
 |
|
Diamonds
~ A Girl's Best Friend
Ah DIAMONDS! Those little sparkly pieces of carbon that everyone seeks after. They are considered valuable by many, but in reality they are simply the crystal form of ordinary carbon, a mineral that is abundant. Since diamonds are created deep in the earth where that carbon gets the required heat and pressure to crystalize, it only follows that other planets where temperatures and pressures are greater would abound in diamonds. Here on Earth value is set because someone controls the amount of diamonds put on the market, though deep in the earth they are abundant. It is only in extinct volcanoes that we can mine them, were because of their weight they are found low in the magma tubes that once created that volcano. So finding diamonds in abundance may not be so great for the jewelry world, but imagine surprizing your gal with a diamond the size of a car There are four planets where it has been confirmed that diamonds are in abundance. These planets are Saturn, Jupiter, Neptune and Uranus. The first article from Berkely U brings a whole new concept to the song "Lucy in the Sky With Diamonds" Menu:
By Robert Sanders, Public Affairs Posted October 6, 1999 Office of Public Affairs at UC Berkeley 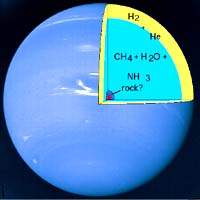 If
experiments at Berkeley are any indication, future
explorers of our solar system may well find diamonds
hailing down through the atmospheres of Neptune and
Uranus. If
experiments at Berkeley are any indication, future
explorers of our solar system may well find diamonds
hailing down through the atmospheres of Neptune and
Uranus.These planets contain a high proportion of methane, which campus researchers have now shown can turn into diamond at the high temperatures and pressures found inside these planets. "Once these diamonds form, they fall like raindrops or hailstones toward the center of the planet," said Laura Robin Benedetti, a graduate student in physics. The team, led by Benedetti and Raymond Jeanloz, professor of geology and geophysics, produced these conditions inside a diamond anvil cell, squeezing liquid methane to several hundred thousand times atmospheric pressure. When they focused a laser beam on the pressurized liquid, heating it to some 5,000 degrees Fahrenheit, diamond dust appeared. They report their experimental findings in the Oct. 1 issue of Science. The demonstration that methane can convert to diamond as well as other complex hydrocarbons in the interiors of giant planets like Neptune hint at a complex chemistry inside gaseous planets and even brown dwarf stars. Brown dwarfs are small, dim stars barely larger than the largest gas giant planets. "This is opening the door to study of the interesting types of chemical reactions taking place inside planets and brown dwarfs," Jeanloz said. "Now that technology is able to reproduce the high pressures and temperatures found there, we are getting much better quality information on the chemical reactions taking place under these conditions." "It is not amazing that chemistry like this happens inside planets, it's just that most people haven't dealt with the chemical reactions that can occur," Benedetti said. "The interior of these planets may be much more complicated that our current picture." A simple calculation, for example, shows that the energy released by diamonds settling to the planet's core could account for the excess heat radiated by Neptune -- that is, the heat given off by Neptune in excess of what it receives from the sun. "What's exciting to us is the application of this high-pressure chemistry to understanding the outer planets," Jeanloz said. "As more planets are found in unexpected orbits around other stars, the effects of internal chemical processes will need to be further clarified in order to obtain a general understanding of planet formation and evolution," the authors conclude in their Science article. Our solar system's other gas giant planets -- Jupiter and Saturn -- may also contain diamonds produced under such conditions, though they contain proportionately less methane than Neptune and Uranus. Based on theoretical calculations, Neptune and Uranus are estimated to contain about 10 to 15 percent methane under an outer atmosphere of hydrogen and helium. Several groups of researchers have suggested that the methane in these planets could conceivably turn into diamond at fairly shallow depths, about one tenth of the way to the center. Nearly two decades ago, a group at Lawrence Livermore National Laboratory shocked some methane and reported the formation of diamond before the stuff evaporated. That group was led by retired scientist Marvin Ross and researchers William Nellis and Francis Ree. Recently theorists in Italy also concluded that diamonds were likely. Benedetti and Jeanloz decided to try the obvious experiment -- squeeze liquid methane and see if they could make diamond dust. "It's really cool to watch," said Benedetti. "When you turn on the laser, the methane turns black because of all the diamonds created. The black diamond specks float in a clear hydrocarbon liquid melted by the laser." It's Raining Diamonds on Neptune and Uranus |
|
Well okay... while the thought of pretty cut diamonds falling from the sky may be on the minds of the media and the populace, in reality what we would find is diamond crystals.  Photo: E. Skalwold - Natural Diamond Crystals 13 Natural Diamond Crystals from 3.18 to 0.05 cts. Benjamin Silliman, Jr. (1816 – 1885) Collection, Cornell University (original label) Professor of Chemistry, Yale University Curator of Yale's mineral collection following his brother-in-law, James Dwight Dana and his father, Benjamin Silliman, Sr. (for whom sillimanite is named). (The first official mineral curator at the Peabody was George Jarvis Brush in 1866). In mineralogy, diamond (from the ancient Greek αδάμας – adámas "unbreakable") is a metastable allotrope of carbon, where the carbon atoms are arranged in a variation of the face-centered cubic crystal structure called a diamond lattice. Diamond is less stable than graphite, but the conversion rate from diamond to graphite is negligible at standard conditions. Diamond is renowned as a material with superlative physical qualities, most of which originate from the strong covalent bonding between its atoms. In particular, diamond has the highest hardness and thermal conductivity of any bulk material. Those properties determine the major industrial application of diamond in cutting and polishing tools and the scientific applications in diamond knives and diamond anvil cells. |
|
'Diamond rain' falls
on Saturn and Jupiter
By James Morgan Science reporter, BBC News  Image Source: DIAMOND PLANETS Diamond rain could be "the most common precipitation in the Solar System" the authors say. Diamonds big enough to be worn by Hollywood film stars could be raining down on Saturn and Jupiter, US scientists have calculated. New atmospheric data for the gas giants indicates that carbon is abundant in its dazzling crystal form, they say. Lightning storms turn methane into soot (carbon) which as it falls hardens into chunks of graphite and then diamond. These diamond "hail stones" eventually melt into a liquid sea in the planets' hot cores, they told a conference.The biggest diamonds would likely be about a centimetre in diameter - "big enough to put on a ring, although of course they would be uncut," says Dr Kevin Baines, of the University of Wisconsin-Madison and Nasa's Jet Propulsion Laboratory. He added they would be of a size that the late film actress Elizabeth Taylor would have been "proud to wear". "The bottom line is that 1,000 tonnes of diamonds a year are being created on Saturn. People ask me - how can you really tell? Because there's no way you can go and observe it. It all boils down to the chemistry. And we think we're pretty certain." - Dr Kevin Baines - University of Wisconsin-Madison Continue reading the main story at BBC News |
 Neptune
in Infrared. Image Credit: W.M. Keck II telescope
in Hawaii
The W.M. Keck II telescope in Hawaii, the world's largest infra red telescope captured the above image, (one of the first Keck images taken with adaptive optics technology, which uses rapid mirror adjustments to remove Earth's atmospheric turbulence from the telescope's images), producing unprecedented clarity. Taken just a few months ago, the images far surpass anything that could have been possible using the Hubble Space Telescope. What the images reveal are giant Neptunian storms, with wind speeds of 600 miles per hour. The images show features that could be frozen land masses separated by hydrocarbon seas and lakes. Scientists have discovered that Neptune, pictured above, and Uranus (below) could be covered in massive seas of liquid diamond - which include huge solid diamond chunks similar in size to icebergs "Diamond is a relatively common material on Earth, but its melting point has never been measured," said J. H. Eggert of Lawrence Livermore National Laboratory in Livermore, California. "You can't just raise the temperature and have it melt, you have to also go to high pressures, which makes it very difficult to measure the temperature." Scientists from Sandia National Laboratories, and other groups, successfully melted diamond years ago, but they were unable to measure the pressure and temperature at which the diamond melted. 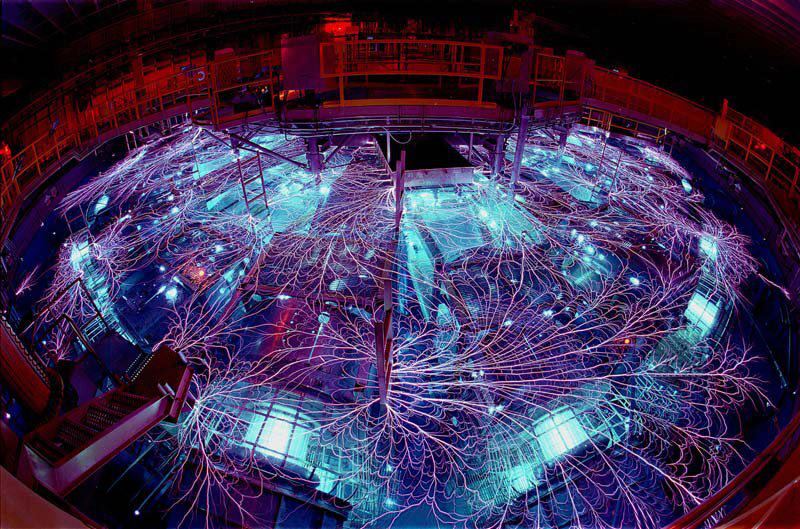 This is the ‘z machine’ above, which liquifies diamond using electricity and pressure, mimicking the environment of the ice-giants. Image Credit: Randy Montoya/Sandia National Laboratories Oceans of liquid diamond, filled with solid diamond icebergs, could be floating on Neptune and Uranus, according to a recent article in the journal Nature Physics. Like ice floating on water, solid diamond floats on liquid diamond. Diamond oceans may be responsible for off kilter planetary tilts and may explain possible liquid diamond oceans on other planets.  The massive oceans on Uranus, pictured, and Neptune could explain some oddities about the planets. Image Courtesy of NASA/Hubble |
Related Papers, Links, Etc
|
|
All material on these pages, unless otherwise noted, is © Pegasus Research Consortium 2001-2019 |
 Webpages © 2001-2019 Pegasus Research Consortium |